Introduction:
Thrombosis is a major cause of mortality and morbidity in myeloproliferative neoplasms (MPN). In patients classified as high-risk for thrombosis, current guidelines recommend the use of cytoreduction therapy and low-dose aspirin (e.g., ASA 81 mg daily) to decrease the risk of thrombotic events. However, use of low-dose aspirin is based on limited evidence and is associated with a significant failure rate.
Thromboprophylaxis with low-dose direct oral anticoagulants (e.g., apixaban 2.5 mg BID) is an appealing option in MPN, specifically when patients carry the JAK2 mutation, based on the pathophysiology of thrombus formation associated with this driver mutation. In other populations, thromboprophylaxis with a low-dose direct oral anticoagulant has been associated with a reduced risk of arterial and venous thrombotic complications, when compared to low-dose aspirin.
Hence, we conducted a pilot trial to assess the feasibility of conducting a randomized controlled trial evaluating the efficacy and safety of apixaban (2.5 mg BID) compared to aspirin (81 mg daily) to prevent thrombotic complications in patients with JAK2-positive MPN (JAK2MPN).
Methods:
This was a multi-centre, prospective, open-label, blinded endpoint pilot trial (NCT04243122), conducted in three Canadian centers (Ottawa, Calgary, Sherbrooke). Adult patients with a confirmed diagnosis of polycythemia vera, JAK2-positive essential thrombocythemia or JAK2-positive pre-fibrotic myelofibrosis (per local clinical definitions) requiring low-dose aspirin for thromboprophylaxis were eligible. Both patients with an incident diagnosis of JAK2MPN (defined as diagnosed within 1 year) and patients with prevalent disease were targeted for recruitment. Exclusion criteria included a contraindication to thromboprophylaxis or the need for a specific anticoagulant or non-aspirin anti-platelet agent. Patients with a prior history of venous thromboembolism were eligible if they had completed a minimum of 6 months of anticoagulation therapy. Participants were randomized 1:1 to receive apixaban 2.5mg BID or aspirin 81mg daily, along with cytoreductive therapy (per guidelines). The study treatment duration was 6 months. Follow-up occurred at 3, 6 and 7 months after allocation of the study medication. The primary study outcome for feasibility was the monthly recruitment rate per study site over a 6-month follow-up period. Secondary outcomes included rates of thrombotic and bleeding complications. The total estimated sample size was 44 participants (22 per arm). Due to the COVID-19 pandemic, not all sites were able to start recruitment at the same time and the total recruitment period spanned over 24 months.
Results:
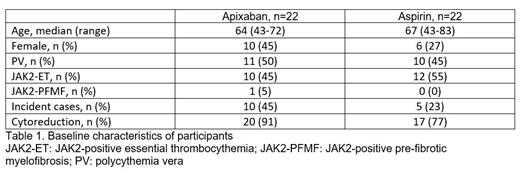
During this 24-month recruitment period, 93 JAK2MPN patients were screened for eligibility. Of these, 69 were eligible and 64% of eligible patients consented to participate. We therefore recruited 44 participants, for a recruitment rate of 0.8 patient per centre per month. Baseline characteristics of participants are presented in Table 1. A total of 29 prevalent cases and 15 incident cases were included. Secondary efficacy, safety, and feasibility outcomes (relating to thrombotic events, bleeding complications, and study compliance) will be available at the ASH Meeting, since all participants will have completed the 6-month treatment duration by October 2023.
Discussion:
Despite the challenges of the COVID-19 pandemic, we have recruited 100% of our target population. Given the chronicity of MPN, most outpatient clinics functioned remotely during the pandemic, precluding recruitment. Nevertheless, by adapting to the realities of the pandemic, we achieved our recruitment target demonstrating feasibility of a larger trial. The strategies adopted during this pilot trial to recruit participants, such as active screening of clinic lists for potential participants and direct communication with MPN specialists, will be applied to our larger scale trial.
Conclusion:
This pilot trial demonstrated the feasibility of conducting a large-scale trial evaluating the superiority of apixaban over aspirin for the thromboprophylaxis of JAK2MPN patients. As JAK2MPN are rare diseases, we have partnered with counterparts in France to conduct an international study on the efficacy and safety of direct oral anticoagulant thromboprophylaxis in JAK2MPN patients.
OffLabel Disclosure:
Siegal:Servier: Honoraria, Other: paid indirectly to my institution; Roche: Honoraria, Other: paid indirectly to my institution; BMS-Pfizer: Honoraria, Other: paid indirectly to my institution; Astra Zeneca: Honoraria, Other: paid indirectly to my institution. Carrier:Sanofi: Honoraria, Other: Payments made to my institution; BMS: Honoraria, Other: Payments made to my institution; Servier: Honoraria, Other: Payments made to my institution; Anthos: Honoraria, Other: Payments made to my institution; Pfizer: Other: Payments made to my institution, Research Funding. Delluc:BMS-Pfizer: Honoraria, Other: Payments made to my institution; LeoPharma: Honoraria, Other: Payments made to my institution.
Apixaban for the thromboprophylaxis of patients with JAK2-positive myeloproliferative neoplasms

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal